10.1 Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcydohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene.
Ans: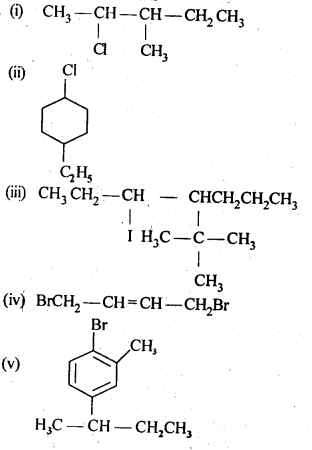
10.2. Why is sulphuric acid not used during the reaction of alcohols with KI?
Ans: KI is expected to give HI on reacting with H2SO4 which will convert alcohols (R – OH) to alkyl iodides (R – I). However, H2SO4 is a strong oxidising agent and it oxidises HI formed during the reaction to I2 which does not react with alcohol.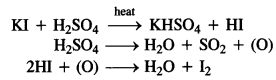
To solve the problem, H2S04 is replaced by phosphoric acid (H3P04) which provides HI for the reaction and does not give I2 as is done by H2S04.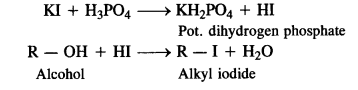
10.3. Write structures of different dihalogen derivatives of propane.
Ans: Four isomers are possible. These are :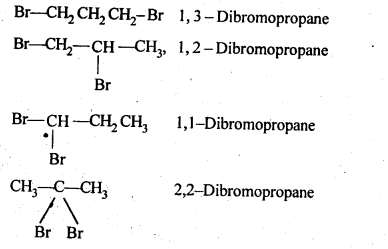
10.4. Among the isomeric alkanes of mdlecular formula C5H12, identify the one that on photochemical chlorination yields
(i) A single monochloride.
(ii) Three isomeric monochlorides.
(iii) Four isomeric monochlorides.
Ans: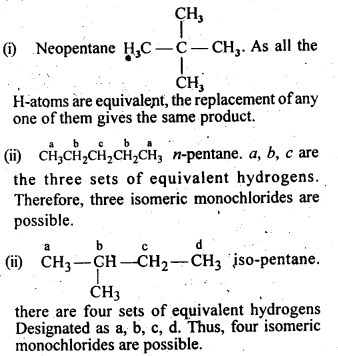
10.5. Draw the structures of major monohalo products in each of the following reactions: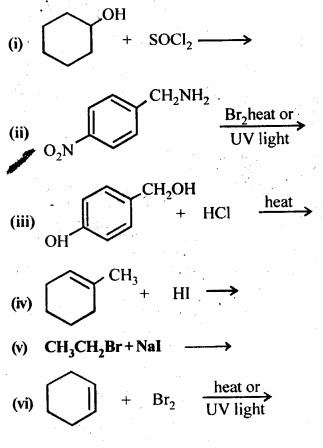
Ans: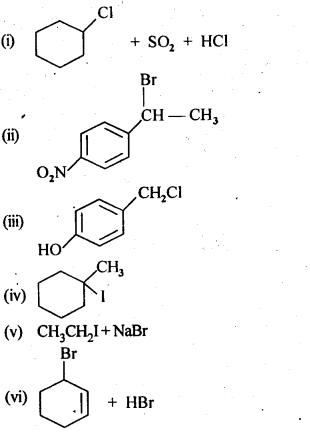
10.6. Arrange each set of compounds in order of increasing boiling points :
(i) Bromomethane, bromoform, chloromethane, dibromomethane
(ii) 1- Chloropropane, isopropylchloride, 1- chlorobutane.
Ans:
(i) The boiling points of organic compounds are linked with the van der Waals’ forces of attraction which depend upon the molecular size. In the present case, all the compounds contain only one carbon atom. The molecular size depends upon size of the halogen atom and also upon the number of halogen atoms present in different molecules. The increasing order of boiling points is :
CH3Cl(chloromethane) < CH3Br (bromomethane) < CH2Br2 (dibromomethane) < CHBr3 (bromoform)
(ii) The same criteria is followed in this case. We all know that the branching of the carbon atom chain decreases the size of the isomer and this decreases its boiling point as compared to straight chain isomer. The increasing order of boiling point is :
(CH3)2CHCl (isopropylchloride or 2-chloropropane) < ClCH2CH2CH3 (1-chloropropane) < ClCH2CH2CH2CH3 (1-chlorobutane)
10.7. Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.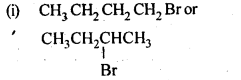
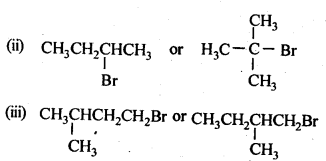
Ans: In SN2 mechanism, reactivity depends upon the steric hindrance around the C-atom carrying the halogen. Lesser the steric hindrance, faster the reaction.
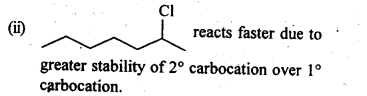
10.8. In the following pairs of halogen compounds, which compound undergoes faster SN1 reaction?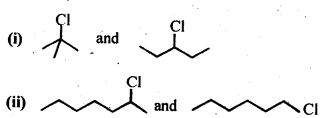
Ans:
10.9. Identify A, B, C, D, E, R and R1 in the following: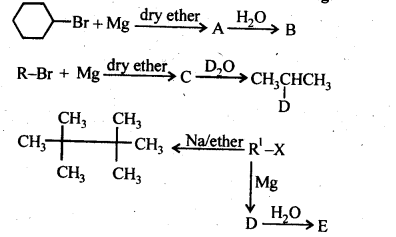
Ans: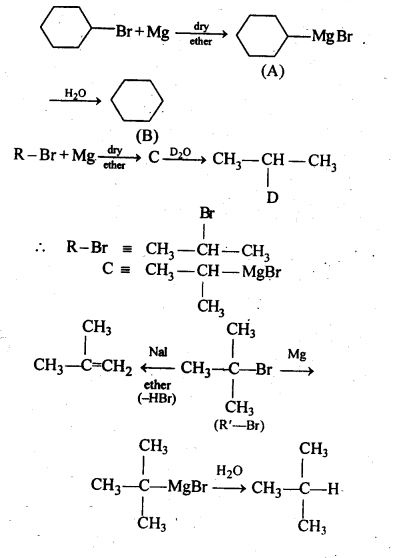
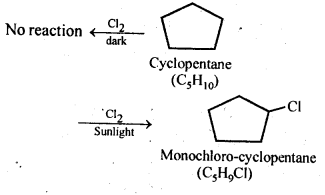
10.10. A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
Ans: The hydrocarbon with molecular formula C5H10 can either a cycloalkane or an alkene. Since the compound does not react with Cl2 in the dark, therefore it cannot be an alkene but must be a cycloalkane. Since the cycloalkane reacts with Cl2 in the presence of bright sunlight to give a single monochloro compound, C5H9Cl, therefore, all the ten hydrogen atoms of the cycloalkanes must be equivalent. Thus, the cycloalkane is cyclopentane.